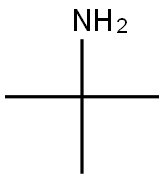
Application of tert-Butylamine
General description
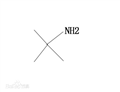
Tert-butylamine appears as a clear colorless liquid with an ammonia-like odor. Flash point 70°F. Less dense (at 6.2 lb / gal) than water. Vapors heavier than air. Toxic oxides of nitrogen produced during combustion. Tert-butylamine is a primary aliphatic amine that is ethylamine substituted by two methyl groups at position 1. It is a conjugate base of a tert-butylammonium. Chemical properties similar to other primary amine.But since the tertiary carbon atom of the three-dimensional effect, have a choice in the reaction.Such as reaction with ethylene oxide generated tert-butyl amino ethano.
Application
Used in synthetic rubber accelerator, pesticide, medicine, surfactant, etc.; The rubber used in the manufacture of rubber accelerator industry, pharmaceutical industry used in the manufacture of rifampicin.
1.Clathrate hydrates preserve active species more stably than the other icy materials and investigation of the behavior of the active species elucidates the physicochemical properties of clathrate hydrates like guest−guest interaction. Color of the tert-butylamine clathrate hydrate changes to blue after gamma irradiation and is bleachable with visible light. The electron spin resonance (ESR) spectrum at 120 K mainly consists of a triplet signal of the C-centered radical NH2C(CH3)2CH2• together with a single signal at g = 2.0008. The latter signal disappears after light exposure. These results indicate that both the blue color and the single ESR signal are derived from trapped electrons in the hydrate. They thermally decay around 140−160 K by the first- order reaction, and the activation energy is 27 kJ/mol. Since tert-butylamine molecules can capture protons due to the high proton affinity, electrons may remain in the hydrate without reacting with protons, making the hydrate blue after gamma irradiation. The long-lived trapped electrons in the tert-butylamine hydrate have an advantage to investigate those in icy materials because tert-butylamine hydrate is nonionic and has a tetra-coordinated host water network like crystalline ice without any substitution for water molecules.
Figure 1 Blue-colored tBA hydrate after gamma-irradiation.
In situ high-pressure crystallization and diffraction techniques have been applied to obtain two very structurally distinct semi-clathrates of the tert-butylamine–water system with hydration numbers 5.65 and 5.8, respectively, thereby considerably reducing a notable hydration gap between the monohydrate and the 71=4-hydrate that results when crystallization space is explored by temperature alone. Both structures can be considered as an intriguing solidstate example of hydrophobic hydration, in which the water network creates wide tert-butylamine-filled channels stabi lized by cross-linking hydrogen bonds. The existence of interconnected channels might also add low hydration structures to a list of potential targets for hydrogen storage. A detailed analysis of the topology of host water and hostguest interactions is reported and extended to those of other hydrates of the compound. This analysis offers new insight into properties of the tert-butylamine–water system and provides some clues as to the occurrence of the sizable number of hydrates of this compound[1,2].
2.(2S,3aS,7aS)-Octahydroindole-2-carboxylic acid benzyl p-toluenesulfonate(4) was obtained by catalytic hydrogenation and condensation-salification from (S)-indololine-2-carboxylic acid(2). Meanwhile, N-[(S) - 1-ethoxycarbonylbutyl]-(S)-alanine(6) was synthesized by one-step method with L-n-valine(5). Compound 4 reacted with compound 6 by condensation, hydrogenation and salt forming to give perindopril tert-butylamine salt(1). In the hydrogenation of 2, rhodium carbon was used as catalyst, and the reaction pressure was decreased from 5 MPa to 2.0 - 3.0 MPa, the reaction time was reduced from 40 h to 4 - 8 h, and the yield was 81%. In the last step, appropriate amount of water was added into hydrogenation solvent and the one-pot method of hydrogenation and salt formation was adopted, which could inhibit the formation of the impurity, ethyl (S)-2-[(3S,5aS,9aS,10aS)-3-methyl-1,4- dioxodecahydropyrazino[1,2-a]indol-2(1H)-yl]pentanoate(7), and the purity of 1 was 99.61%[3].
Synthesis
With tertiary butyl alcohol and urea as raw materials in the sulfuric acid condensation, hydrolysis and butylated urea, reoccupy, sodium hydroxide and 40% of the water, drain, and liquid. alkali.
Safety
Invasive ways: inhalation, eat, percutaneous absorption.Health hazard: inhalation, ingestion or absorbed through the skin may be fatal.To the eyes, skin, mucous membranes and respiratory tract.
Reference
1.Tani A., Koyama S. & Urabe Y. et al., "Blue-colored tert-butylamine clathrate hydrate," J Phys Chem B, Vol.118, No.47(2014), pp.13409-13413.
2.Granero-Garcia R., Falenty A. & Fabbiani F. P., "Dense Semi-Clathrates at High Pressure: A Study of the Water-tert-Butylamine System," Chemistry, Vol.23, No.15(2017), pp.3691-3698.
3Yong-jun jin, Han Zheng, Qian Liangwei such as: "without perindopril split tert-butylamine salt synthesis process improvement", journal of China pharmaceutical industry 10, 2020.
You may like
Lastest Price from tert-Butylamine manufacturers
US $0.00/Kg/Drum2025-04-21
- CAS:
- 75-64-9
- Min. Order:
- 140KG
- Purity:
- 99%
- Supply Ability:
- 1000 Tons
US $0.00/Kg/Drum2025-04-21
- CAS:
- 75-64-9
- Min. Order:
- 140KG
- Purity:
- 99%
- Supply Ability:
- 1000 tons